In nuclear medicine, a radioactive substance is introduced into a patient and finds its way to specific biological targets in the body. Depending on the radioactive properties of the radioisotope, it may emit radiation that can be detected with external detectors to visualise the distribution of the isotopes (SPECT, PET imaging); alternatively, it may emit charged particles like α or β- particles which deposit their energy locally (within μm to a few mm, namely from the size of a cell to the size of a metastasis), thereby only destroying cells located nearby, e.g. to treat a cancer with targeted radionuclide therapy (TRNT).
Out of the more than 3,000 different radioisotopes that scientists have synthesized in the laboratory, only a handful are regularly used for medical procedures, mostly for imaging, though the interest in TRNT has been growing in the last few years as illustrated with the marketing of Lutathera® to treat advanced prostate cancer. One of the main limits to the development of novel radio-medicinal products is the access to radionuclides during the development and early biomedical research phases. Within PRISMAP – The European medical radionuclides programme, we aim at enabling this development phase by providing access to novel radioisotopes of high purity grade for medical research.
Production of radioisotopes
The radioactive elements that are used in nuclear medicine are not available naturally and must be synthesised in the laboratory. There are two main paths: neutron irradiation in a nuclear research reactor or proton or alpha irradiation with a particle accelerator. The size and energy of the particle accelerator determines which radioisotope can be produced: small, compact machines are found in many hospitals, providing access to the radioisotopes used today. However, higher-energy machines are needed to produce novel radioisotopes currently not available.
Purification of radioisotopes
When producing those novel radioisotopes, new challenges appear: the co-production of unwanted radioactivity which affects the quality of the medicinal product, may induce adverse effects to a patient, and can cause serious difficulties to waste management in a hospital environment. As such, novel purification techniques are required. Within PRISMAP – The European medical radionuclides programme, we shall develop techniques based on physical mass separation and radiochemistry to achieve high purity radioisotope production that is appropriate for medicinal products.
Access and translational research
In order to support the ongoing research across Europe and beyond, immediate access to novel radioisotopes will be provided by PRISMAP – The European medical radionuclides programme. A single-access platform has been established via our website where the production and support capabilities are presented.
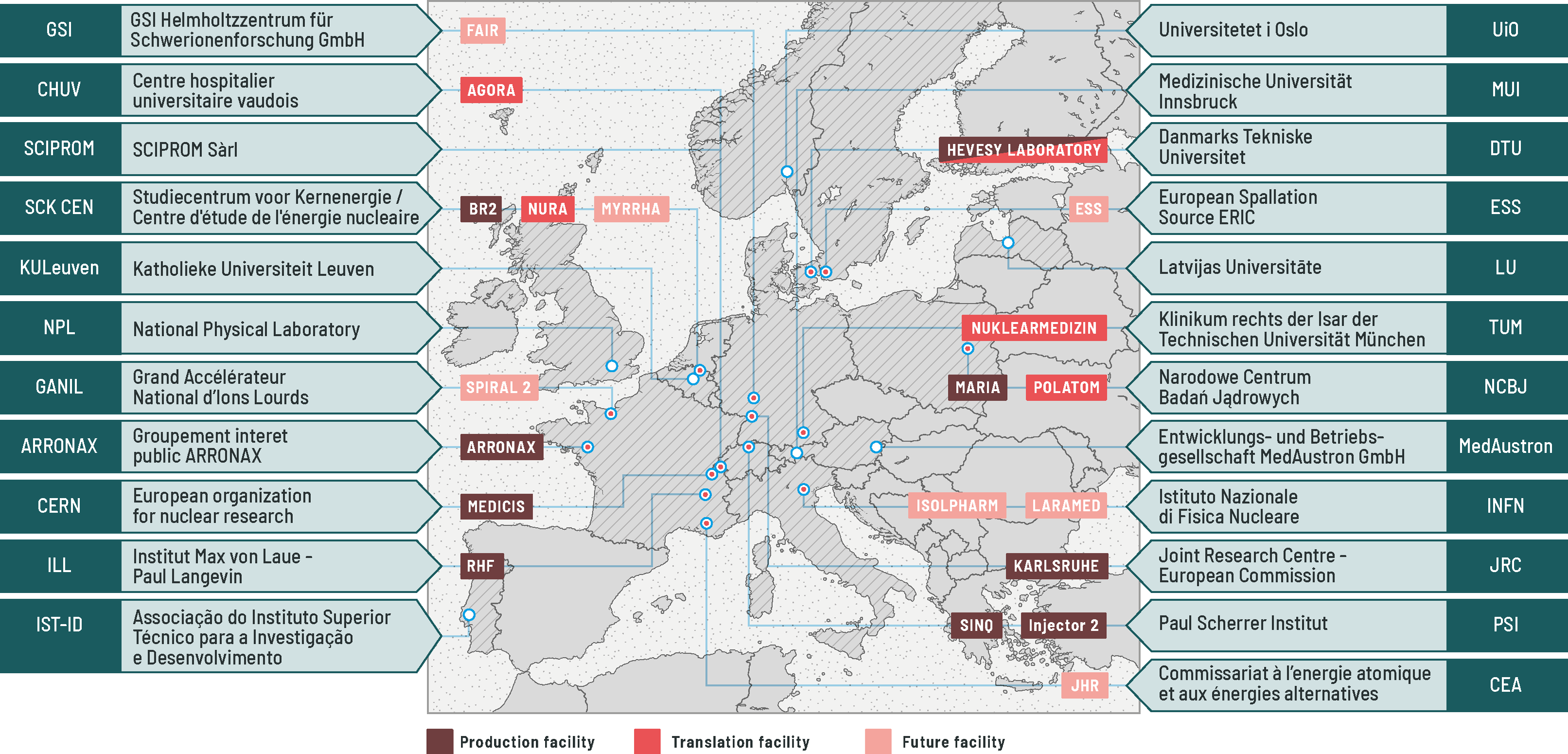
A network of world-leading, European facilities, including nuclear reactors, medium- and high-energy accelerators, and radiochemical laboratories, has been established to offer the broadest catalogue of radioisotopes for medical research. Mass separation is available at the CERN MEDICIS facility to provide the physical separation of isotopes of an element. This is completed by a network of biomedical research facilities who may host external researchers to perform their research close to the production facility when the isotopes are not suitable for long transport to their institution, or when the European licencing for novel radioisotopes has not yet been obtained.
Access to radioisotopes and associated facilities will be granted on an excellence selection basis, by applying for access to radioisotopes and, if necessary, to the complementary biomedical facilities, via our online access platform. A selection panel consisting of experts in the fields of radioisotope production, molecular imaging and radionuclide therapy will select the best projects from the applicants. The first call for proposals will be launched before the end of 2021 for applications in the first quarter of 2022. It will be open to any interested party.
Looking towards the future
In the fast-evolving landscape around nuclear medicine, PRISMAP – The European medical radionuclides programme is also turned towards the future. The European Commission has expressed its commitment to tackle societal impact on cancer through the Europe’s Beating Cancer Plan, and in particular the SAMIRA Action Plan unveiled earlier this year, including the establishment of a European Radioisotope Valley Initiative. Through the PRISMAP consortium of 23 academic and research institutions across Europe, development towards the upscaling of the production of these novel radioisotopes will be investigated, in the form of novel production technology, new purification methods, and proof-of-concept investigations showing the development of new treatments from test bench to patient care, directly feeding this European-wide plan.
As a consortium serving a starting researcher’s community, we are looking to become a more established community and to welcome new facilities to enlarge our capabilities. Novel facilities are on the horizon, such as the Jules Horowitz Reactor in CEA Cadarache (France), the ISOL@MYRRHA mass separator facility at SCK CEN (Belgium), the new SPES accelerator complex in the INFN’s Legnaro National Laboratories (Italy), the European Spallation Source in Lund (Sweden), and finally both the new SPIRAL2 facility at GANIL (France) that has recently accelerated its first beams and the FAIR facility in GSI (Germany) which construction is progressing. Those new facilities will directly benefit from the findings within PRISMAP towards increasing the production capacity across Europe.

New data will be generated and compiled towards the immediate and smooth adoption of the novel radioisotopes in medical environments though collaboration between research hospitals and metrology institutes (e.g. the National Physical Laboratory in Teddington, UK). All the new findings will be used towards creating new teaching material for professionals in the various fields of this multi- disciplinary domain, as well as for training the next generation of professionals and advising the European Commission on these emerging radioisotopes.
Nuclear medicine research is a truly multidisciplinary approach, and to move forward, we need to build bridges between physicists, engineers, radiochemists, inorganic chemists, structural biologists, clinicians, medical physicists, dosimetrists, pharmacologists, and oncologists. PRISMAP – The European medical radionuclides programme, will certainly support the implementation of a multidisciplinary working concept in practice.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 101008571 (PRISMAP – The European medical radionuclides programme).